|
High Paying
US Jobs, Economic
Empowerment
&
Revitalization
of
States through Federal Power |
"Critical Field"
Technology Innovation
&
Maximized Profits on
R&D and Investments |
Prevention of Biochemical
and Nuclear Proliferation
&
Homeland Security
Technologies |
Chemotherapy Treatment with No Side
Effects
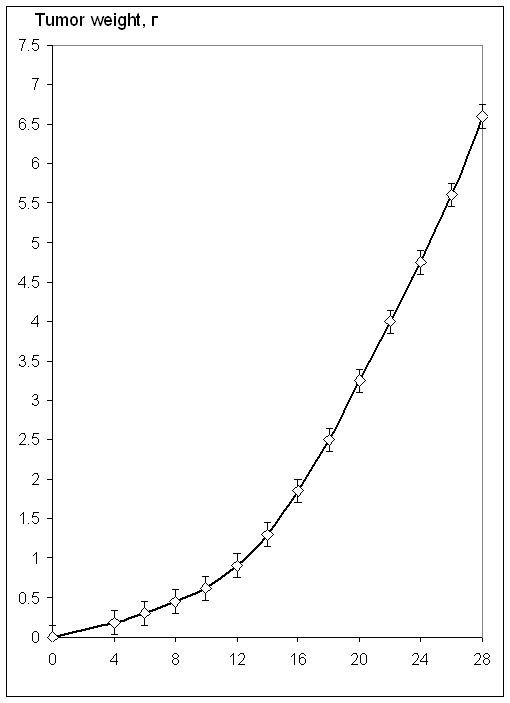
A breakthrough in chemotherapy of using ultra-low doses (ULD)
of existing tumor-inhibiting drugs with no side effects has been proven in
animal experiments on mice. The standard dosage of doxorubicin (as an example of
anti-tumor antibiotics) for the treatment of carcinoma is 1.4*10-3 M (8 mg/kg).
Doxorubicin in this amount is very harmful not only to the tumor but to the
patient and his health.
This drug and chemotherapy drugs in general target rapidly
dividing cancer cells. However, other cells that also divide rapidly include
blood cells, cells that line the digestive tract, and cells in hair follicles.
Unfortunately, these healthy cells may also be affected by the chemotherapy
drugs, resulting in side effects such as infections, tiredness, temporary hair
loss, and mouth sores.
By lowering the amount of doxorubicin slightly, the side
effects are also decreased but so are the effectiveness of inhibiting cancer and
the life expectancy of the patient. Using a revolutionary phenomena, it has been
proven that if the amount of the drug is lowered to a molecular level 10-5 to
10-20 M more then a thousand times less then the standard dosage, the side
effects are gone, the life expectancy of the patient is the same as with
standard dosage, and the inhibition of the drug is even more successful with ULD
then with standard dosage.
We have found that doxorubicin in ULD inhibits the growth of
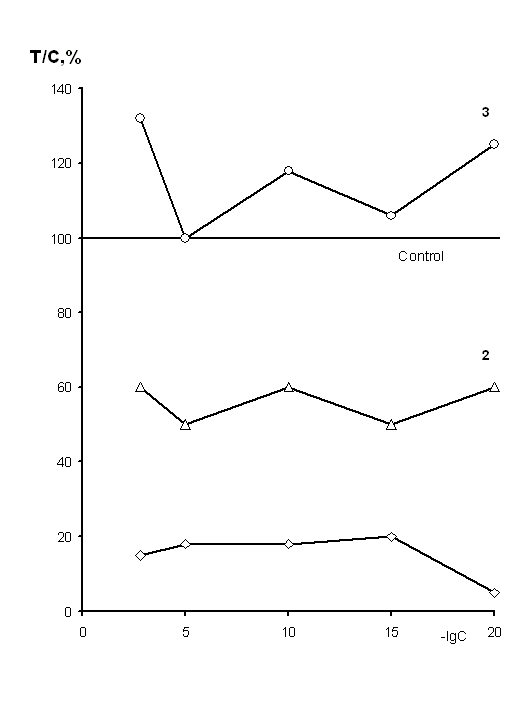
carcinoma Luis by 95% with the deepest inhibition occurring at the dose levels
of 10-20 M. The standard dose used today inhibits the growth of the tumor by
85%. The length of life of the animals treated with ULD increased by 28% and
standard therapeutic dose by 33% compared to the control group.
The amount of drug is absolutely safe and will be easily
approved by the FDA.
There are over eight million Americans alive today who have a
history of cancer and about 83 million; i.e., one in three Americans, who will
eventually develop cancer.
Human clinical protocols have been prepared and will be
applied to patients who can not be treated with a regular dosage because of a
heart condition, or whose treatment can not be continued due to the inability to
survive additional treatment.
The sensitivity of experimental tumor systems
to ultra low doses of adriamycin.
Ostrovskaya L.A.,
Bluchterova N.V., Korman D.B.,
Fomina М.М.,
Rikova В.А.Burlakova
E.B.
Institute of biochemical physics
Russian Academy of Science,
Moscow, Russia
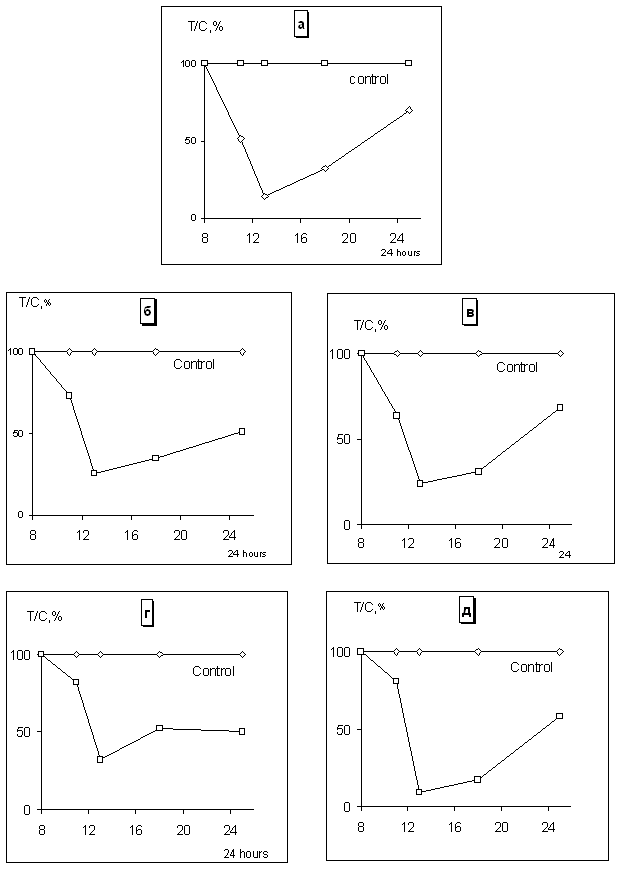
Biological effect of adriamycin in standard (10-3 M) and ultra
low doses (10-5 10-20 M) against some signal
animal tumor systems Lewis lung carcinoma, 755 adenocarcinoma,
B 16 melanoma, Ehrlich carcinoma and
L 1210 leukemia was studied. The all models were very sensitive to the action of
the drug in standard dose. Solid tumors growth inhibition at 80 95% as well
as increasing in life span of mice with L 1210
leukemia at 86% in comparison with control and surviving of animals with
Ehrlich carcinoma had been revealed. It had been shown that the drug in the area
of ultra low doses occured the following effects:
-
inhibition of
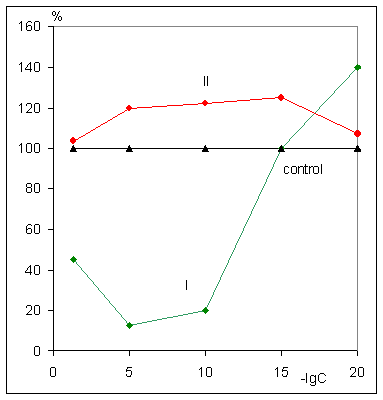
Lewis lung carcinoma growth at 80-95% compared to control after administration
of the all tested ultra low doses;
-
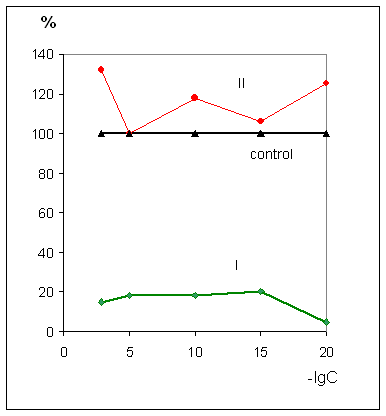
increasing of the life span of the animals with Ehrlich carcinoma and L
1210 leukemia at 86-123% and 6 23%, correspondingly, upon the action of all
tested ultra low doses;
-
inhibition of B 16 melanoma growth at 50 and 70% after administration of
the drug in doses 10-20 M and 10-5 M, correspondingly as
well as deceleration of 755 carcinoma growth at 40% compared to control after
action of the drug in the dose 10-20 M;
-
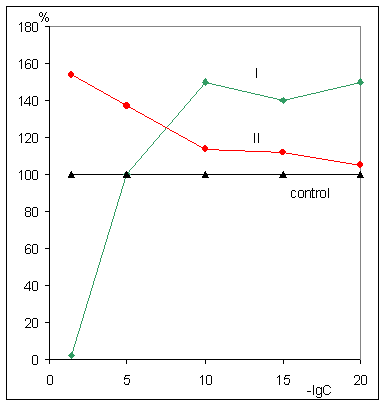
stimulation of the B 16 melanoma growth at 20% relative to control after
10-10 M dose injection and enhancement of tumors sizes on 20-60%
above control levels as a result of treatment of mice with 755 carcinoma by the
drug in such ultra low doses as 10-5 and 10-15 M.
So, it was found that all tested tumor systems revealed certain
sensitivity to some ultra low doses of the drug. At the same time it was shown
that adriamycin in ultra low doses displays an alternative character of its
biological effect, directivity of which varied according with the dose level and
tumor strain.
Summary
The ability of some drugs to exert biological activity in ultra low doses
reveals the new approaches to the treatment of various diseases, in particular
for chemotherapy of malignant tumors.
The goal of this
study was evaluation of biological effects of cytostatics with different
mechanism of action scheduled in ultra low doses. Levels of these doses
were ten and more times less then levels of standard therapeutic doses of
tested drugs.
Antitumor drugs -
adriamycin, cyclophosphamide, methylnitrosourea were tested against
Lewis lung carcinoma in wide spectrum of ultra low doses. The drugs were
applied with single i.p. injection on the next day after tumor transplantation
in the following ultra low doses 10-5, 10-10, 10-15,
10-20 M, as well as in standard therapeutic doses ( 10-2
10-3 M).
It was found that
cytostatics in ultra low doses may reveals the antitumor activity against animal
tumor systems
In particular,
adriamycin in ultra low doses was strong effective against Lewis lung carcinoma.
Tumor growth inhibition was practically the same
after administration of the drug in ultra low or standard doses: 70
95% or 85% in comparison with control, correspondingly.
Upon the whole it
was shown that cytostatics in ultra low doses display biological effects,
directivity of which varied according with the
kind of the drug, the
dose level and the tumor strain.